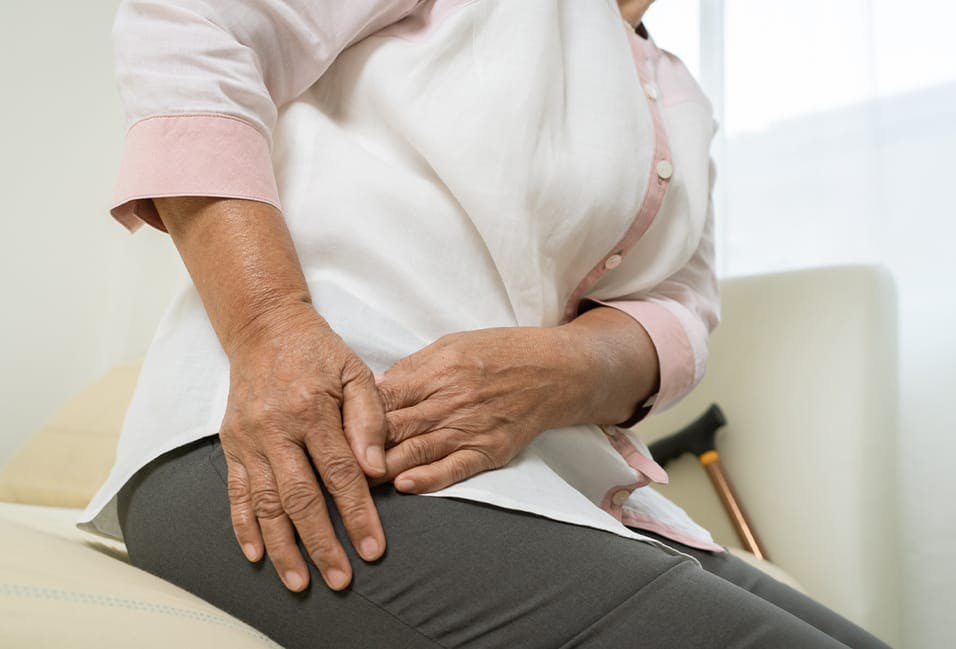
You may be entitled to compensation for medical expenses, injuries, and other damages if you are suffering because of Exactech joint replacement surgery.
What is the Exactech Recall?
Exactech is a leading medical device company specializing in the manufacture of hip, ankle, and knee replacement devices. The Florida-based company issued a recall for over 147,000 implants used during hip, knee, and ankle replacement surgeries in February 2022. The recall was made throughout the United States for devices manufactured between 2004 and the present day.
The recall is for those devices that were packaged in non-conforming or out-of-specification vacuum bags. It was discovered that these devices may be associated with the following:
- Pain
- Inflammation
- Joint failure